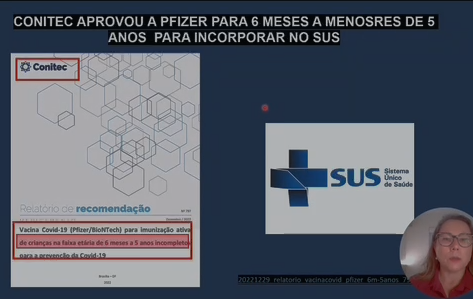
How did Pfizer Covid manage to enter the market so quickly, if it takes decades to develop a vaccine? The manufacturer merged the testing phases 1/2/3, which is not correct, and obtained ANVISA registration with the product without completing phase 3. In the ANVISA registration report for Pfizer baby, the agency admits that conclusive data on efficacy and safety were pending, such as the risks of myocarditis and pericarditis in the age group of 6 months to under 5 years. The manufacturer also did not have safety data in immunocompromised children and what the rare adverse events were. When Pfizer children’s vaccine was launched, the director of ANVISA warned about the risk of adverse events and the need to seek immediate medical attention.