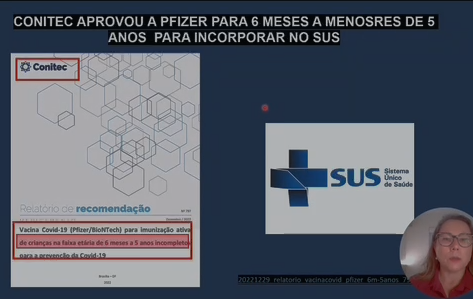
Contrary to what is said, Pfizer Baby and Pediatric never received FDA registration. They only had emergency use authorization. Recently, in the US, they were removed from the recommendation for the children’s calendar by the CDC. However, here in Brazil, ANVISA registered this product (Baby and Pediatric) without information on the report on the completion of the safety and efficacy tests. Therefore, it is still in the experimental phase.